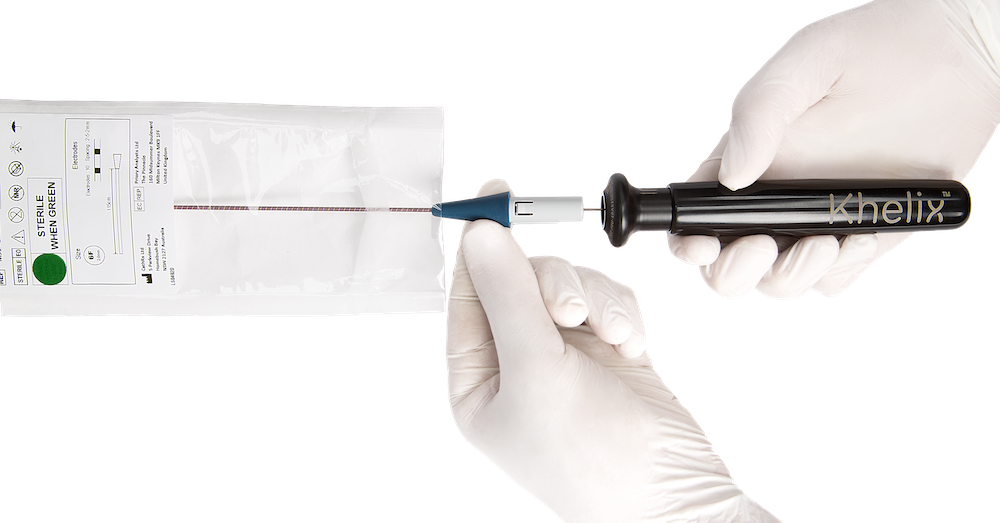
CathRx is fast becoming a global leader in ablation technology. We have harnessed next-generation technology to develop a patented, advanced & modular ablation platform. Our platform is a unique multi-indication ablation solution that has been designed in partnership with the world’s leading physicians and brought to life by our engineering team, headquartered in Sydney, Australia. We believe catheter therapeutics are the future of healthcare, and our mission is to utilise our platform to change the lives of patients & their families all around the world.